AlveoShield™
(Investigational New Drug)
Pioneering a New Path in Neonatal Lung Protection
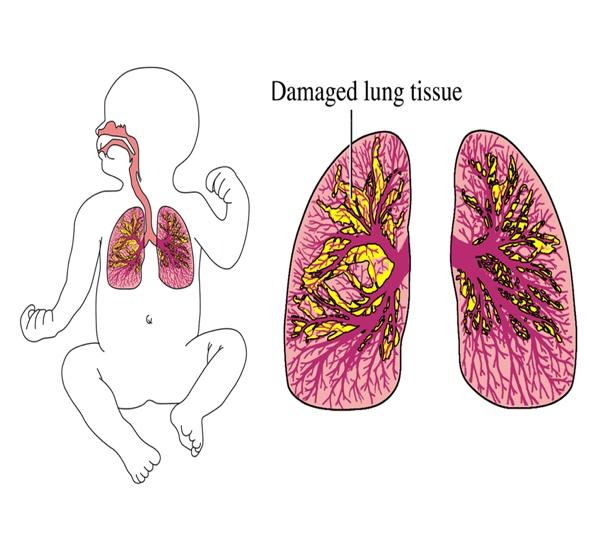
Bronchopulmonary Dysplasia, A Critical Clinical Unmet Need
Bronchopulmonary dysplasia (BPD) is one of the most severe and costly complications of preterm born infants. It affects approximately 30-40% of premature infants each year and frequently leads to lifelong respiratory challenges, prolonged hospitalizations, and major financial strain on families and healthcare systems.
Despite decades of research, to date there is no approved therapy exists to prevent or treat BPD.
CanVeer is focused on advancing AlveoShield™ as a preventative/ early mitigation therapy for BPD, partnering with clinicians, researchers, and industry leaders to transform neonatal respiratory care.
Bronchopulmonary dysplasia (BPD) is a chronic lung/ respiratory disease that affects preterm newborns who are born with their lungs underdeveloped and will require assisted ventilation (oxygen therapy) to survive. Whether the oxygen therapy is administered through mechanical ventilation or non-invasive methods, because the preterm newborns’ lungs are underdeveloped the oxygen pressure often “overstretch” the internal lungs’ structure (bronchi and alveoli), causing inflammation and irreversible or long-term damage to the inside lining of the airways and the blood vessels around them. The inflammation and damage to the lungs tissue causes scares (dysplasia) which compromise the normal development of the lungs predisposing to BPD.
Infants who develop BPD may suffer from a variety of symptoms including trouble in feeding, pulmonary hypertension, lifetime breathing difficulty, delayed speech, heart defects, high vulnerability to viral and bacterial lung infections
The BPD Challenge in Numbers:
- 180,000+ preterm infants annually in North America require prolonged respiratory support
- 40% will develop BPD, leading to lifelong health burdens, and perhaps life threatening consequence
- Approximately $500K+ in estimated lifetime healthcare costs per affected child
- Zero disease-modifying therapies currently exist
AlveoShield™ Therapeutic Potential
AlveoShield™ (new investigational drug) is First-in-Class Therapeutic for Prevention
and Treatment of bronchopulmonary dysplasia (BPD). AlveoShield™ is CanVeer
Biopharma’s Flagship therapeutic candidate and the foundation of our intellectual
property portfolio. It represents a new class of non-invasive neonatal respiratory
therapeutics specifically designed to prevent and treat BPD, a disease with no approved pharmacologic therapy.
The AlveoShield™ Advantage: Ahead-of-the-game Start, Built on Solid Science:
The AlveoShield™ development is built on a robust scientific foundation:
- 1. Characterized target & biological pathway
- 2. Defined biomarkers for monitoring patient response to treatment
- 3. Completed Proof-of-Concept studies in postmortem neonate tissue & in-vitro models
- 4. Completed Pre-Clinical efficacy studies in juvenile neonate animal models
AlveoShield™ Clinical Relevance:
- Addresses a major clinical unmet medical need with no current drug treatment.
- Potential to reduce Neonatal intensive care units (NICU) stays, improve long-term lung function, and significantly lessen healthcare costs.
- Targets a validated biological pathway associated with lung injury and impaired lung development in preterm infants.
- Designed for safety and efficacy in the neonatal population, where developing safe, non- invasive therapeutic options are extremely challenging.
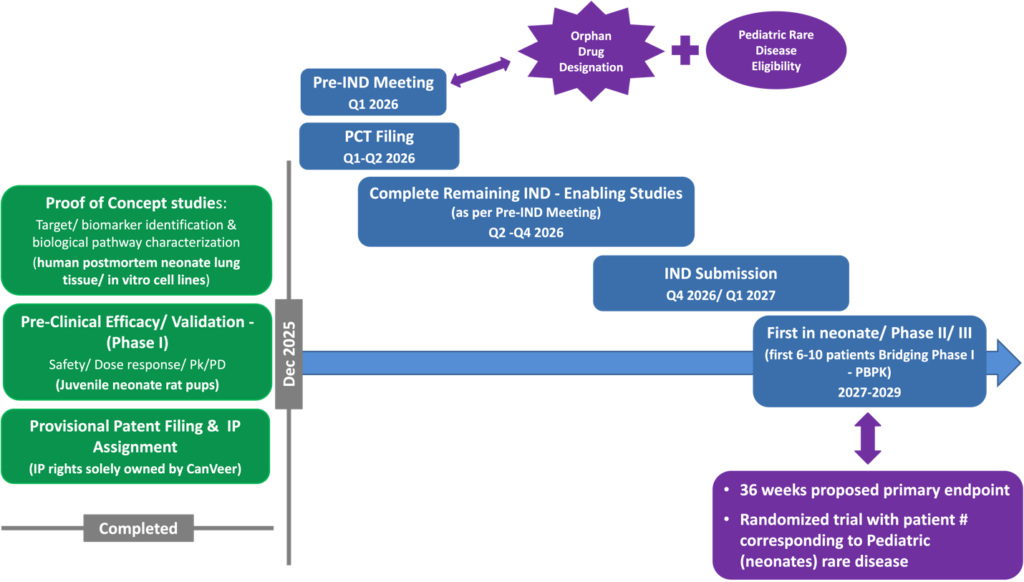
AlveoShield™ Path to the Clinic
Near-term Milestones for AlveoShield™ Clinical Development
Our goal is to bring AlveoShield™ through clinical development and partner with leading
pharmaceutical and healthcare organizations for global commercialization.
